Bexotegrast (PLN-74809): PSC
Bexotegrast as a Potential Treatment for Primary Sclerosing Cholangitis (PSC)
Our lead product candidate, bexotegrast (PLN-74809), is an oral, small molecule, dual-selective inhibitor of the αvβ6 and αvβ1 integrins being developed for the treatment of primary sclerosing cholangitis (PSC).
The αvβ6 and αvβ1 integrins are expressed at very low levels in normal tissues but are upregulated in the liver tissues of PSC patients. Both integrins serve as activators of TGF-ß, leading to increased collagen production and, ultimately, fibrosis in these tissues. By blocking the activation of TGF-ß by both αvβ6 and αvβ1, we believe bexotegrast may slow and potentially halt the progression of fibrosis in this patient population.
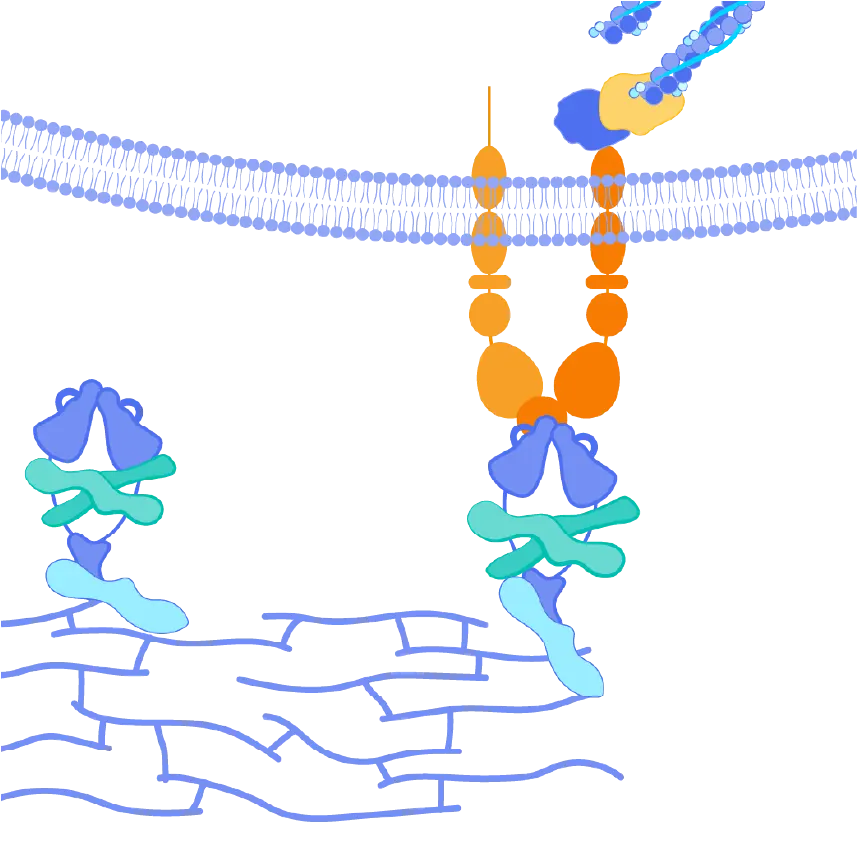
Bexotegrast (PLN-74809) – Pliant’s Tissue-Specific Approach to Treating Fibrosis
INTEGRIS-PSC
INTEGRIS-PSC is a Phase 2a, randomized, dose-ranging, double-blind, placebo-controlled trial evaluating the safety, tolerability, and pharmacokinetics of bexotegrast administered over 12 weeks in patients with PSC. Patients were enrolled in doses of 40 mg, 80 mg, 160 mg, or 320 mg and stratification based on use of ursodeoxycholic acid (UDCA).
STATUS: Enrollment complete
Program Highlights
- INTEGRIS-PSC, a global Phase 2a trial in PSC, interim 12-week data reported
- INTEGRIS-PSC 12-week high-dose data expected first quarter 2024
- Currently no FDA-approved therapies
Development Status
- Granted Orphan Drug Designation from FDA and EMA
- Granted Fast Track Designation from FDA