Bexotegrast (PLN-74809): IPF
Bexotegrast as a Potential Treatment for Idiopathic Pulmonary Fibrosis (IPF)
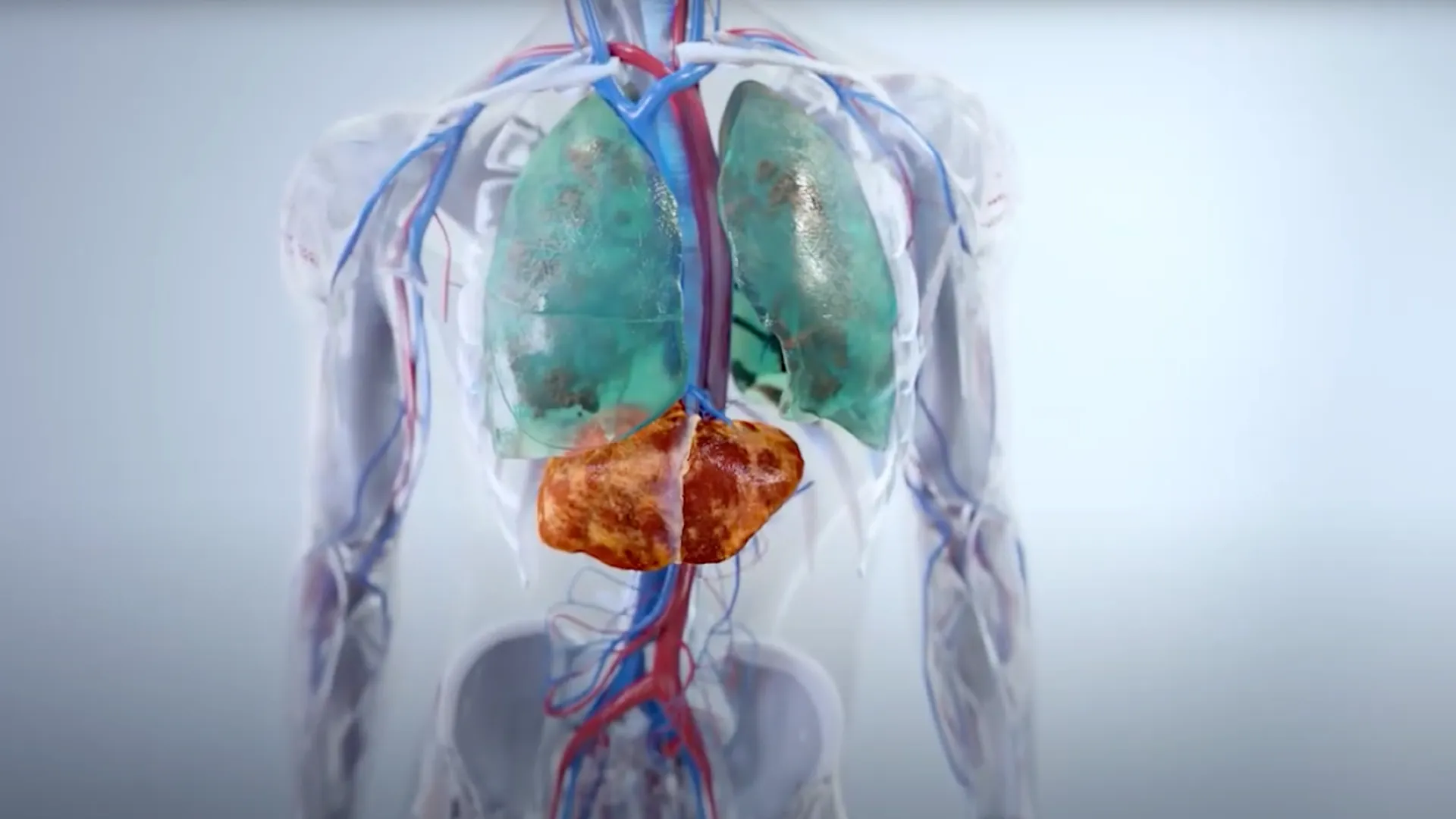
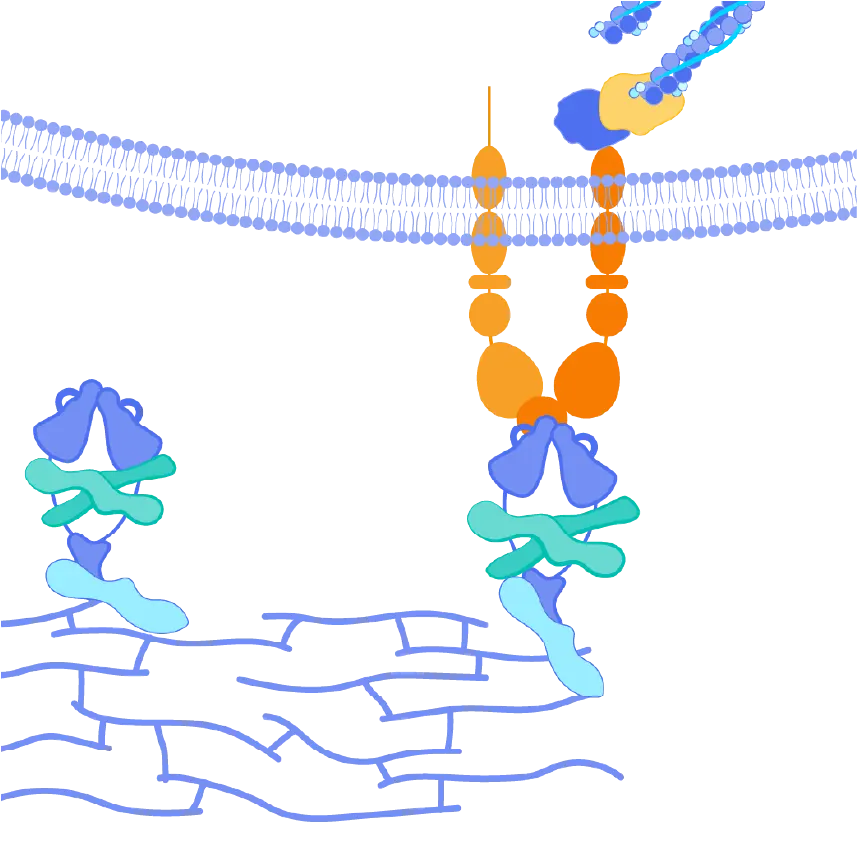
Our lead product candidate, bexotegrast (PLN-74809), is an oral, small molecule, dual-selective inhibitor of the αvβ6 and αvβ1 integrins. It is being developed for the treatment of idiopathic pulmonary fibrosis (IPF).
The αvβ6 and αvβ1 integrins are expressed at very low levels in normal tissues but are upregulated in the pulmonary tissues of IPF patients. Both integrins serve as activators of TGF-ß, leading to increased collagen production and, ultimately, fibrosis in these tissues. By blocking the activation of TGF-ß by both αvβ6 and αvβ1, we believe bexotegrast may slow and potentially halt the progression of fibrosis in this patient population.
Bexotegrast (PLN-74809) – Pliant’s Tissue-Specific Approach to Treating Fibrosis
BEACON-IPF is a global clinical trial evaluating the investigational drug bexotegrast for the treatment of IPF.
STATUS: Enrolling
To learn more, please visit BeaconIPF.com.
Program Highlights
- INTEGRIS-IPF, a global Phase 2a trial in IPF, data reported
- Currently only two existing FDA-approved therapies for IPF, both shown modest slowing of disease progression
- Medical need remains high
Development Status
- BEACON-IPF, a global clinical trial in IPF, is enrolling
- Granted Orphan Drug Designation from FDA and EMA
- Granted Fast Track Designation from FDA